What are the E and Z isomers of pent-2-ene?
1 Answer
Apr 10, 2016
See below.
Explanation:
The formula of pent-2-ene is
Each double-bonded carbon atom has an
In (
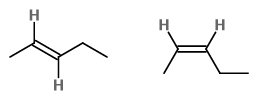
In (
See below.
The formula of pent-2-ene is
Each double-bonded carbon atom has an
In (

In (