What are the strongest bonds in water?
1 Answer
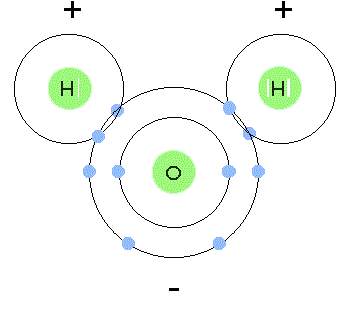
The intramolecular polar covalent bonds in the water molecule are the strongest bonds in each water molecule.
Explanation:
In the water molecule,
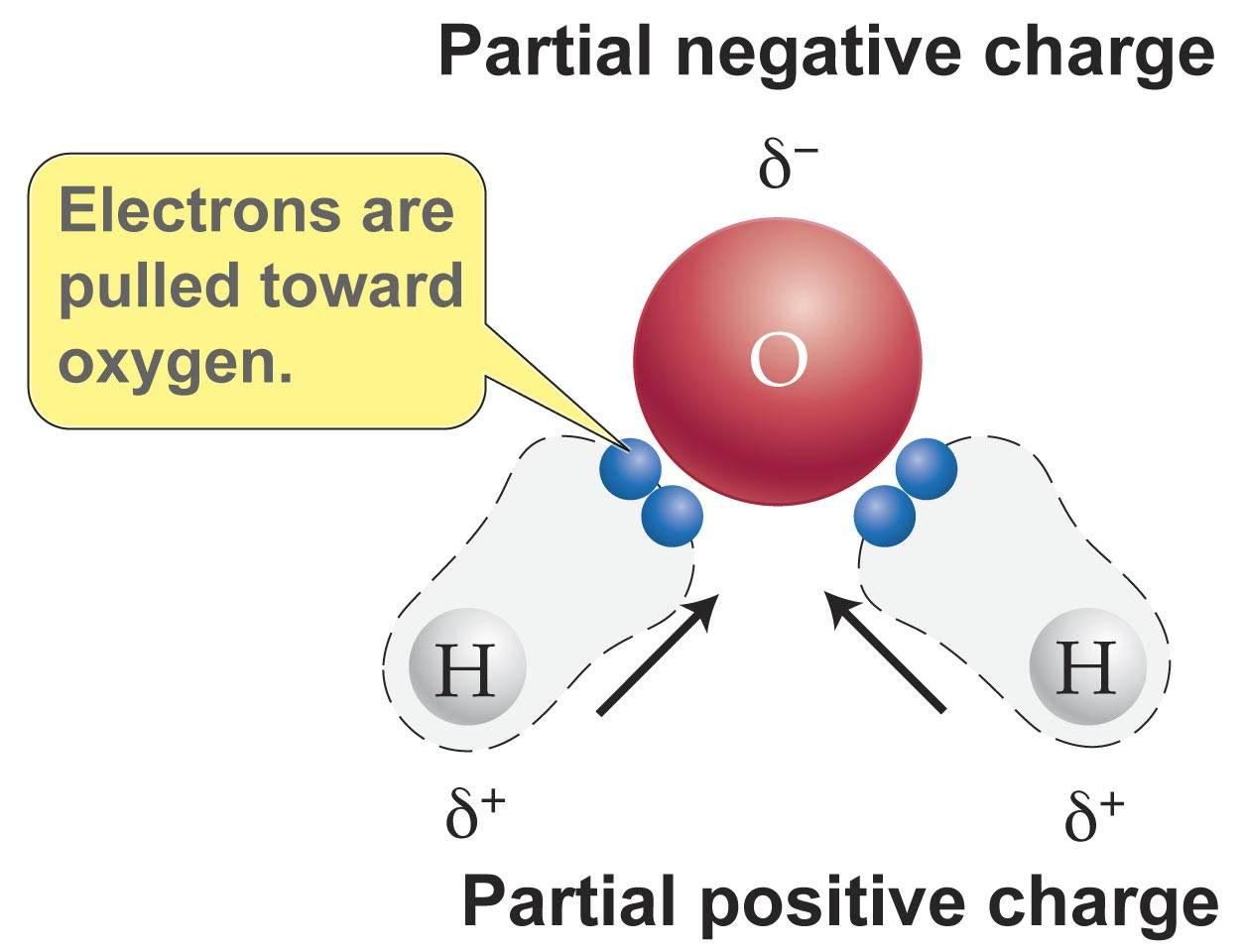
These polar covalent bonds are intramolecular (meaning within the molecule) and are the strongest bonds in water.
The diagram below illustrates the polar covalent bonds between O and H.
The diagram below illustrates the polarity of the water molecule, and the unequal sharing of electrons by O.