What are the symbols with oxidation numbers for sulfuric acid? What is the formula?
1 Answer
Dec 5, 2016
I can only assume what you are trying to ask in your question, but sulfur has an oxidation number of
Explanation:
In sulfuric acid, the molecular formula is
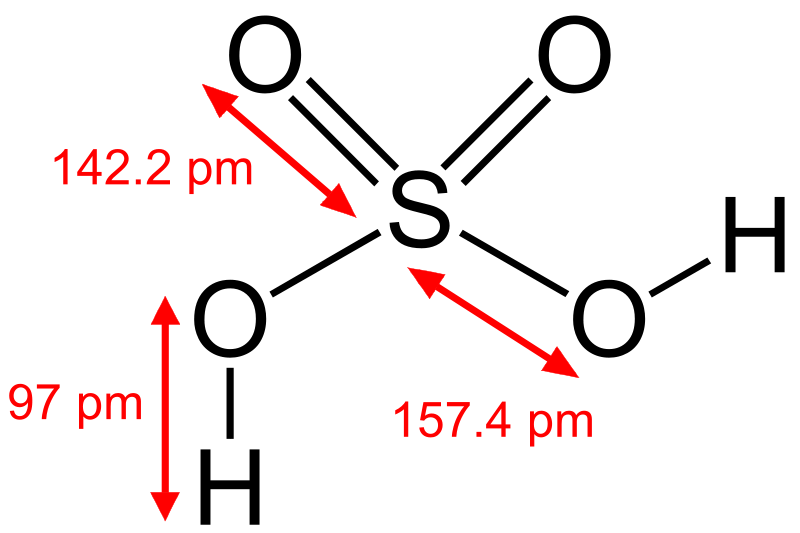 .
.
As you can see, sulfur has donated six electrons, each oxygen atom has accepted two electrons and each hydrogen atom has donated one electron.

