What are two characteristics of a molecule that make it suitable for analysis by infrared spectroscopy?
I know that one of them is that it must have a dipole but is there another?
I know that one of them is that it must have a dipole but is there another?
1 Answer
You are right that it has to have a dipole (not necessarily a net dipole). I'm not sure what other specific requirements you may be wondering about, but I can list several, and maybe one of them is what you are looking for.
REQUIREMENTS FOR VISIBILITY IN THE IR REGION
The main properties of a molecule that allow it to be analyzable via IR spectroscopy are:
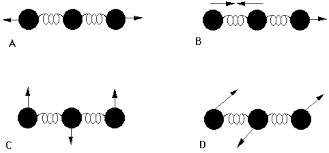
#1)# It can experience a change in dipole moment, whether it is induced or permanent. We then must have that it is heteronuclear, if it is diatomic (therefore,#"N"_2# ,#"F"_2# , etc. are invisible in the IR).
#2)# It should have resonant frequencies that are in the infrared frequency range of#100 - 4000# #"cm"^(-1)# .
#3)# Ideally, it should be not overly soluble in a nonpolar solvent, which is ideal since many analyses are carried out using a solvent such as#"CS"_2# or#"CCl"_4# . If something is too soluble, one may saturate or overload the spectrometer.
CHANGE IN DIPOLE MOMENT
Requirement
A somewhat tricky example is
Even though it is symmetrical, it can stretch both oxygens in the same direction (as in
RESONANT FREQUENCIES IN THE IR RANGE
This is usually satisfied automatically, just due to the general strengths of chemical bonds, but it couldn't hurt to check. The fundamental frequency can be estimated using the force constant
#bb(tildeomega = 1/(2pic)sqrt(k/mu))# ,where:
#tildeomega# is the fundamental frequency in#"cm"^(-1)# .#c# is the speed of light,#2.998 xx 10^(10) "cm/s"# .#k# is the force constant in#"kg/s"^2# .#mu = (m_1m_2)/(m_1 + m_2)# is the reduced mass of the two atoms in the bond, where each mass is in#"kg"# .
As an example, I randomly found on a quick Google search that
#tildeomega = 1/(2pi*2.998 xx 10^(10) "cm/s") sqrt(("2385 kg/s"^2)/((12.011*15.999)/(12.011 + 15.999) xx 10^(-3) "kg"/"mol" xx "mol"/(6.0221413 xx 10^(23) "molecules"))#
#~~ "2429 cm"^(-1)#
and the literature value for the fundamental frequency is
This is found to be the strongest peak in its IR spectrum:
which you can estimate to be near
SOLUBILITY IN A NONPOLAR SOLVENT
Nice IR solvents that are commonly used are
This kind of consideration is not necessary, but being too soluble may make it frustrating to get a spectrum without overloading the spectrometer. Being "somewhat" soluble in nonpolar solvents is probably fine.