What can the electronegativity difference between the atoms in a molecule of HCI can be used to determine?
1 Answer
See below
Explanation:
The
If the
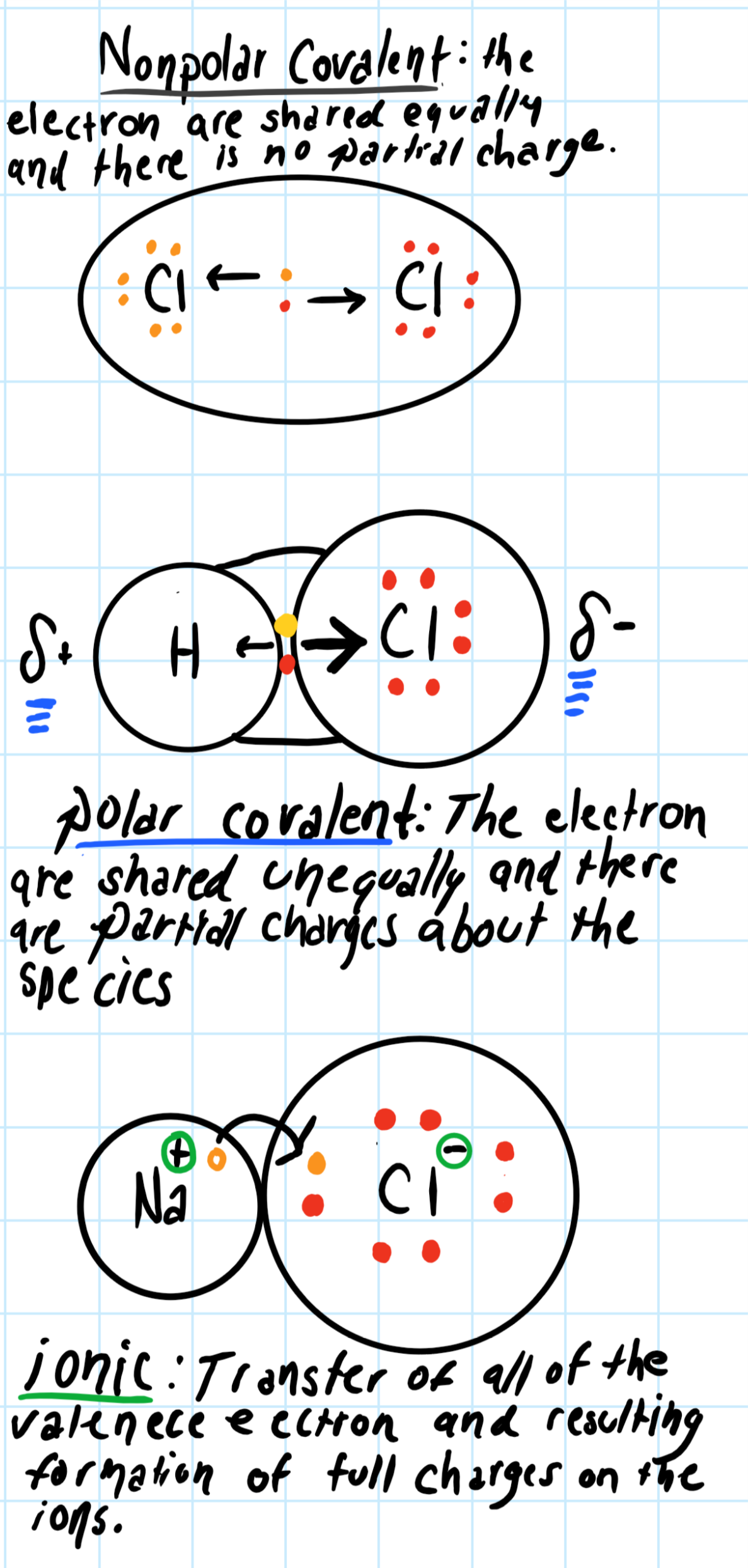
One species is always going to be at least slightly more electronegative than the other unless they are the same species.
In our case, for determining the
For the
And when using the Pauling scale of electronegativity values to find the electronegativity difference, usually, if:
So using the Pauling scale and subtracting the electronegativity values of
tells us that the
Since we know that the bond is polar covalent, this tells us how the electron are shared about the species. Try to imagine how the electron are interacting about the species:
Consider the graphic below: