What do Van der Waals forces do?
1 Answer
see below.
Explanation:
Van der Waals forces (are also called London Dispersion Forces ) are the only possible intermolecular interactions (or forces) that exist between element or non polar molecules.
The are electrostatic attractions between temporary dipoles (or induced dipoles ) that form in atoms of non polar molecules.
As electrons move around the nucleus, a momentary nonsymmetrical distribution of charge will result in producing a temporary dipolar arrangement of charge.
This, in turn, will affect the distribution of charge in the neighboring atom. This means that an instantaneous dipole will induce a similar dipole in the neighboring atom as seen in the figures below.

Image source: Reproduced from Bozeman science.
The same phenomenon could happen also in non polar molecules at the molecular orbital level.
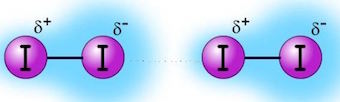
Image source: http://www.learnnext.com
Note that larger molecules or bigger size atoms will have high polarizability which will facilitate the formation of the induced dipoles.

Image source: https://www.utdallas.edu
The larger the hydrocarbons chains, the larger the surface area and the stronger the Van der Waals forces. This can be easily seen from the difference in boiling points of hydrocarbons.
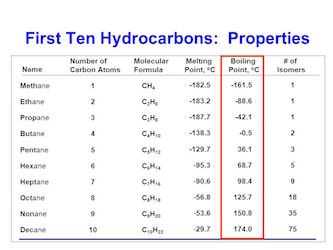
Image source: images.slideplayer.com

