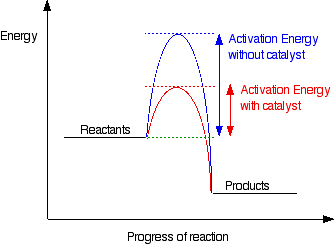
What does an enzyme decrease in order to increase the rate of a chemical reaction?
1 Answer
Nov 4, 2016
An enzyme or other catalyst lowers the activation energy to increase the rate of a reaction.
Explanation:
Many chemical reactions do not occur rapidly enough on their own to be useful, or in the case of living systems to be capable of sustaining life. So catalysts lower the energy required to start the reaction. They do this without being used up so they are always available, although in some cases they may be used up in secondary reactions.