What does #OsO_4#/ #tBuOOH#/#HO^-#or #KMnO_4#/#NaOH#/0°C do?
1 Answer
Jan 3, 2015
Both reactants convert alkenes to vic-diols.
-
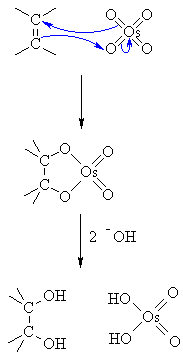
The OsO₄ is a catalyst. It reacts with the π electrons of the alkene in a syn addition to form a cyclic osmate ester.
-
The OH⁻ hydrolyzes the ester. This forms the cis-diol and H₂OsO₄.
-
The t-BuOOH oxidizes the H₂OsO₄ and regenerates the OsO₄ catalyst: t-BuOOH + H₂OsO₄ → t-BuOH + OsO₄ + H₂O
**

As with OsO₄, the reaction goes through a cyclic ester to form a cis diol.
In this case, the MnO₄⁻ is a reactant, not a catalyst, because the MnO₄⁻ is not regenerated.

