What is #OsO_4# (osmium tetroxide)?
1 Answer
Osmium tetroxide is an oxide of osmium.
It has many uses, despite the fact that the abundance of Os in the earth's crust is only 1.5 ppb by mass.

OsO₄ is colourless and has a chlorine-like odour. Most samples appear yellowish because of contamination by yellow-brown OsO₂.
OsO₄ is tetrahedral and nonpolar.

Some of its physical properties are:
- melting point = 40°C
- sublimes at room temperature
- boiling point = 130°C
- solubility in water = 6.2 g/100 mL
- solubility in CCl₄ = 375 g/100 mL
The most common use of OsO₄ in organic chemistry is to convert alkenes to vic-diols.
The mechanism involves a concerted cis addition to form a cyclic osmate ester, which then hydrolyzes to form the diol.
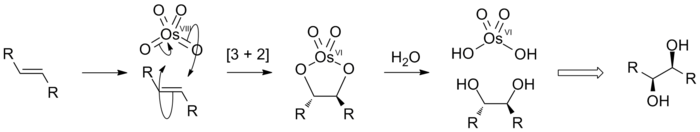
OsO₄ is usually used in small amounts as a catalyst. Reactants such as H₂O₂ are added to regenerate the OsO₄.
OsO₄ is expensive (over $200/g) and highly toxic. The permissible exposure limit is only 2 µg/m³ over 8 h.
OsO₄ can even diffuse through plastic, so it must be kept in glass containers and in a fume cupboard.

