What does the atomic number of an element tell us?
1 Answer
Jun 21, 2018
The number of massive, positively-charge nucular particles...
Explanation:
And thus it tells us the IDENTITY of the element by defining its atomic number.
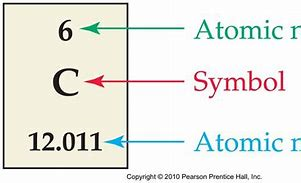
And of course for the NEUTRAL atom, the atomic number is ALSO the number of electrons, that are conceived to orbit the nuclear core. Why so?
The nucleus also contains, neutrons, massive particles of ZERO electronic charge, but which engage with nuclear protons in the strong nuclear force, an attractive force, the which at impossibly short nuclear ranges, is STRONGER than the electrostatic force of repulsion, and binds atomic nuclei together...

