What is a sigma bond example?
1 Answer
Apr 13, 2016
If we follow the convention that:
- The internuclear axis of a linear molecule is the
#z# axis - The internuclear axis of a nonlinear molecule is the
#y# axis
...then any two orbitals overlapping head-on is a
Thus, noting that there are other possibilities, the following orbital combination examples would work (excluding
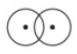
EX:
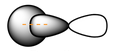
EX:
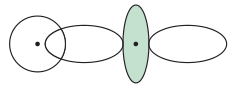
EX:
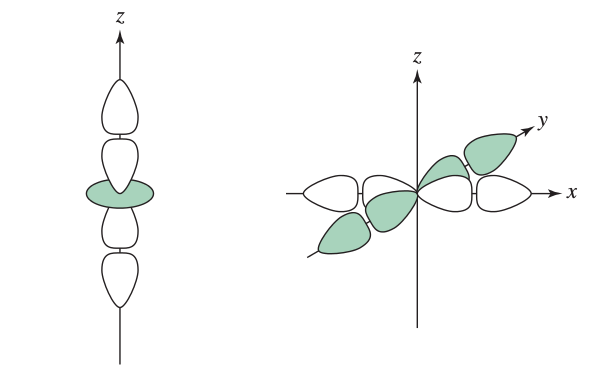
(yes, these are
EX:
#"NH"_3# 's#"N"-"Cr"# bond in#["Cr"("NH"_3)_2("NCS")_4]^(-)# (#p_y + d_(z^2)# )#"NCS"# 's#"N"-"Cr"# bond in#["Cr"("NH"_3)_2("NCS")_4]^(-)# (#p_y + d_(x^2 - y^2)# )

EX:
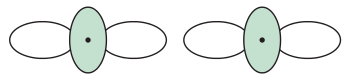
EX:

