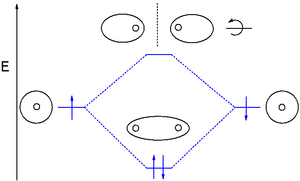
What is a sigma bond? How does the overlap of two 1/2 filled 1s orbitals produce a sigma bond?
1 Answer
A
EFFECTIVE ORBITAL OVERLAP BALANCES ATTRACTIVE-REPULSIVE INTERACTIONS
While making a chemical bond, there is a balance between the nuclear(A,B) repulsion energy and the nucleus(A/B)-electrons(B/A) attraction.
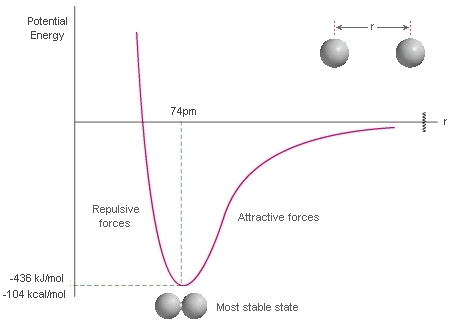
When that is just right, i.e. the potential energy is minimized, the orbitals have overlapped effectively.
ORBITAL OVERLAP CAN BE IN-PHASE OR OUT-OF-PHASE
When two
The electron density is the probability distribution for finding electrons in a certain region of space. The higher the density in a spot, the easier it is to find the electron in that spot.
In addition, when one of the