What is anisotropy in nmr?
1 Answer
Well this is a feature of NMR spectroscopy that profs use to batten down and confuse students with, and it implies the directional dependence of a species under a given analysis.
Explanation:
In NMR spectroscopy, possibly the best example of anisotropy occurs with the benzene molecule, in which the
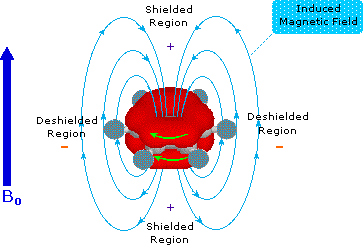
Now in the diagram consider the behaviour of the
So a current, i.e. movement of electrons, is induced around the benzene ring. This OPPOSES the applied field with respect to the interior of the ring, but with respect to the exterior of the ring, it REINFORCES the applied magnetic field. The result? Well, protons on an aryl ring are said to be deshielded with respect to conventional protons, and their resonance occurs significantly downfield. The physical manifestation of this deshielding is the appearance of the protons of benzene at
And now consider the performance of another aromatic species in the NMR experiment, i.e. 18-annulene, which is another aromatic molecule with 18 delocalized
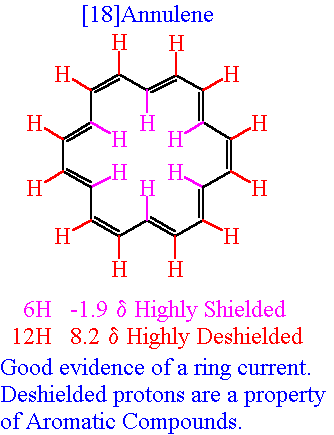
The 12 protons EXTERIOR to the ring are deshielded like the aryl protons. However, the protons INTERIOR to the ring are STRONGLY SHIELDED, in that the induced magnetic field OPPOSES the applied field, with the result that a signal is observed at
I acknowledge that there is a whole lot of info here to digest.....

