What is going on with the alkyl migration step in the Schmidt reaction mechanism on a ketone?
I am asking this for a friend. :) I'm also going to answer this myself.
I am asking this for a friend. :) I'm also going to answer this myself.
1 Answer
The Schmidt reaction for a ketone involves reacting with
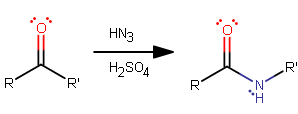
The mechanism is quite interesting, and goes as follows:
- The carbonyl oxygen is protonated, since it has high electron density. This catalyzes the reaction so that hydrazoic acid can attack on the next step.
- Hydrazoic acid behaves almost like an enolate would, and nucleophilically attacks the carbonyl carbon.
- The mechanism continues towards forming an imine, so we protonate the
#"OH"# to form a good leaving group. - The imine forms and the
#"H"_2"O"# leaves. - A proton is taken from the iminium nitrogen.
- Here is where the alkyl migration occurs. Note that this slightly resembles a more typical concerted 1,2-alkyl migration (
#"E"2# ) process when a primary carbocation would otherwise form during the elimination reaction.Since the
#"N"-"N"# #sigma# bond is weak (only slightly stronger than a peroxide#"O"-"O"# #sigma# bond, by about#"15 kJ/mol"# ), it favorably breaks, and the larger/bulkier#R"'"# group migrates onto the imine nitrogen. Since the#"N"-"N"# bond favorably breaks, it frees up the#sigma^"*"# antibonding orbital in the imine nitrogen and allows the alkyl group to donate into it. It is interesting though unclear why the larger#R"'"# group is the one that migrates, apparently "regardless of its nature" (pg. 15). - The iminium carbocation intermediate forms, which I suspect is unexpected, though it has been proven to form (cf. here, pg. 15). Water can then easily behave as a nucleophile and bond.
- To perform the forward reaction we cannot eliminate the water from the molecule (that would reform the intermediate), so we deprotonate it.
- Tautomerization ensues for the hydroxylimine intermediate, grabbing a proton from the recently protonated water.
- Finally, the mechanism is concluded when the deprotonation occurs to form the amide product.
Alkyl migration is quite fun to run into, but actually is not unique to this mechanism.
Another quick example of alkyl migration is in the 1,1-insertion reactions within transition-metal-carbonyl complexes where an alkyl group is cis to a
In this case the alkyl group migrates, and the
Come to think of it, there is another interesting example of alkyl migration in hydroboration!
See if you can spot it: