What is instantaneous rate of reaction? and how we can determine it?
1 Answer
Here's what I get.
Explanation:
The definition
Suppose we have a reaction that forms a product
A graph of the concentration
The instantaneous rate of reaction is the slope of the line (the tangent to the curve) at any time
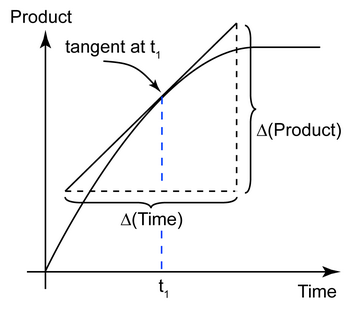 Rate
Rate
(From SPM Chemistry)
How do we determine it?
We draw the best tangent to the line that we can and extend it to convenient points on the axis that will make it easy to calculate the value of
For example, the graph below shows the volume of carbon dioxide released over time in a chemical reaction. Find the instantaneous rate of reaction at t = 40 s.
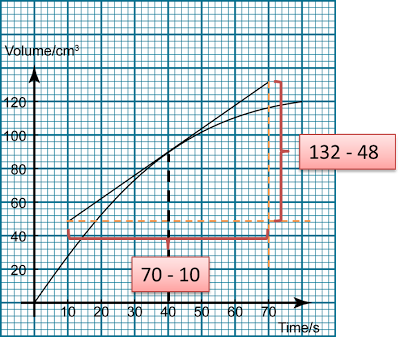 4.bp.blogspot.com
4.bp.blogspot.com

