What is the atomic number of phosphate?
2 Answers
Sep 11, 2016
None, phosphate is not an element
Explanation:
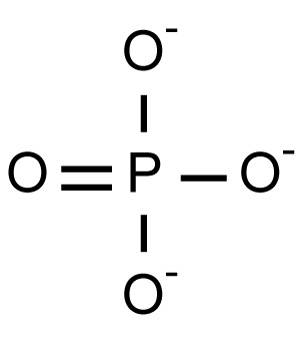 Phosphate is an anion with the chemical formula
Phosphate is an anion with the chemical formula
Sep 11, 2016
Phosphate anion is a polyatomic ion with a formal charge of
Explanation:
Phosphates are the salts of
The atomic number of phosphorus is


