What is the difference between structural isomer, geometric isomers, and enantiomers?
1 Answer
Well, they all got the same chemical formula....
Explanation:
Structural isomers have the same chemical formula (and typically they are organic compounds)...but different connectivity. For example...butane,
As the formula becomes bigger, with more carbons, the number of isomers is magnified...
On the other hand, geometric isomers each have the SAME
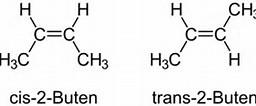
Just to persuade you that this does make a difference, the boiling point of
And the third type of isomerism, occurs when the connectivity is the same..and yet the spatial properties of the molecule gives the result that the structure generates two or more stereoisomers...and typically this occurs when a carbon atom in the molecule has 4 different substituents...such a carbon can generate left-handed, and right-handed enantiomers that can only be equated by a mirror plane.

