What is the electron geometry and molecular geometry for # SCl_2#?
1 Answer
Nov 13, 2016
The electron geometry is tetrahedral and the molecular geometry is bent.
Explanation:
To determine the geometries, you must follow a series of steps.
Step 1. Draw the Lewis structure.

Step 2. Use VSEPR theory to determine the electron geometry.
According to VSEPR theory, four electron groups will point towards the corners of a tetrahedron.
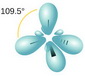
(From BC Open Textbooks)
The electron geometry is tetrahedral.
Step 3. Determine the molecular shape.
When describing the shape of a molecule, we ignore the lone pairs and look only at the bonds.
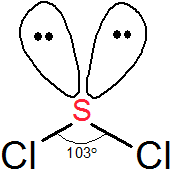
The two

