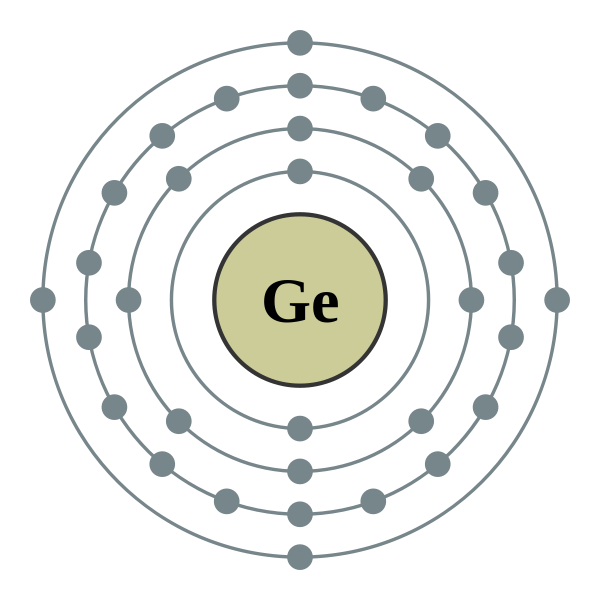
What is the ground state electron configuration of the element germanium?
1 Answer
Jan 13, 2015
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So,
An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation. The closest noble gas to come before Ge in the periodic table is Argon (Ar), which means that the electron configuration we want is