What is the molecular geometry of H2O? Draw its VSEPR structure.
1 Answer
Bent
Explanation:
First, draw the Lewis structure of water:
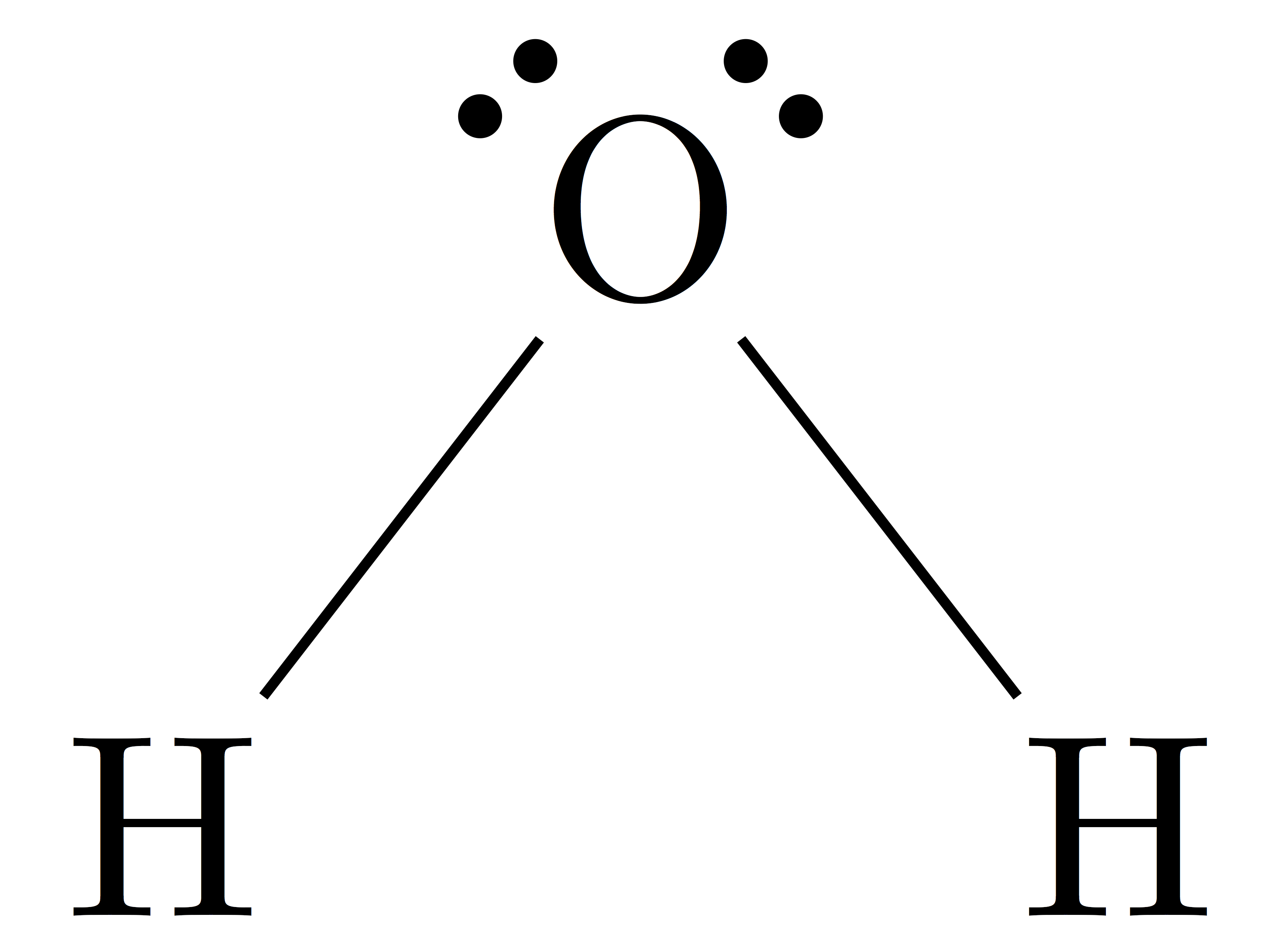 Wikipedia
Wikipedia
Since the O atom has two bonds and two lone pairs, its steric number is 4. The following table shows VSEPR structures based on a compound's steric number and the number of lone pairs:

Since the steric number is 4 and there are two lone pairs, water has bent geometry.


