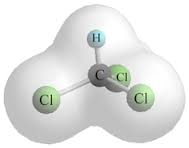
What is the molecular geometry, or shape, of chloroform #CHCl_3#?
1 Answer
Jul 16, 2017
Tetrahedral Parent and Geometry =>
Explanation:
From the formula given
Non-bonded pairs =
Valence electrons = 1H + 1C +3Cl = 1(1) + 1(4) + 3(7) = 26
Substrate electrons = 1H + 3Cl = 1(2) + 3(8) = 26
Non-bonded
#e^(-)# pairs =#(V_e - S_e)/2# =#(26 - 26)/2# = 0
Bonded