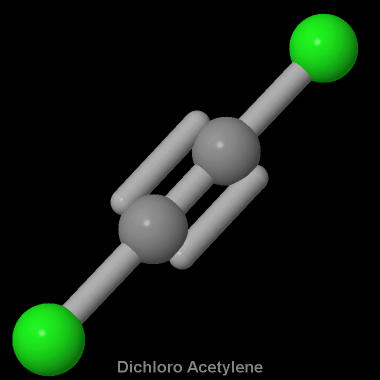
What is the molecular shape of C2Cl2?
1 Answer
The molecular shape of the
Starting with its Lewis structure, the
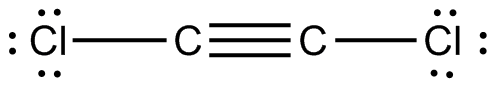
So, the two carbon atoms are bonded to the two chlorine atoms through a single bond, and through a triple bond to each other. The triple bond accounts for
The 3 lone pairs around each of the two chloride atoms add up to
According to VSEPR Theory, the molecular geometry of the molecule will be linear, since each of the two carbon atoms has a coordination number and a steric number equal to 2, with a bond angle of