What is the radioactive particle released in the following nuclear equation #""_74^159"W" -> ""_72^155"Hf" + ? #
1 Answer
Explanation:
The nuclear equation given to you describes the decay of tungsten-159 to hafnium-155
#""_ (color(white)(a)74)^159"W" -> ""_ (color(white)(a)72)^155"Hf" + ""_Z^A?#
Your goal here is to find the values of
Here
In any nuclear reaction, charge and mass must be conserved. This means that you can write
#159 = 155 + A -># conservation of mass
#color(white)(a)74 = color(white)(a)72 + Z -># conservation of charge
Solve these two equations to find
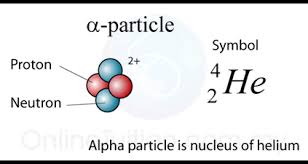
#A = 159 - 155 = 4#
#Z = 74 - 72 =2#
You can thus say that the unknown particle will have a mass number equal to
The balanced nuclear equation will thus look like this
#color(green)(|bar(ul(color(white)(a/a)color(black)(""_ (color(white)(a)74)^159"W" -> ""_ (color(white)(a)72)^155"Hf" + ""_2^4alpha)color(white)(a/a)|)))#