What is the set of four quantum numbers that represent the electron lost to form the #"K"^(+)# ion from the #"K"# atom?
1 Answer
Explanation:
Your goal here is to find the values of the four quantum numbers that are used to describe the location and spin of an electron inside an atom.
Start by writing the electron configuration of a neutral potassium atom,
#"K: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(4)color(darkgreen)(s)^color(blue)(1)#
When potassium forms the potassium cation,
As you can see, the electron lost when potassium becomes as ion comes from the
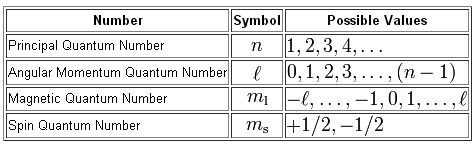
The principal quantum number,
#n = color(red)(4)#
The angular momentum quantum number,
#l=0 -># designates the s-subshell#l=1 -># designates the p-subshell#l=2 -># designates the d-subshell#l=3 -># designates the f-subshell
In your case, the electron comes from the
#l = 0#
The magnetic quantum number,
#m_l = 0 -># designates the s-orbital
Finally, the spin quantum number,
#m_s = +1/2 -># designates spin-up
Therefore, the quantum number set that describes potassium's valence electron looks like this
#color(green)(|bar(ul(color(white)(a/a)color(black)(n=4, l=0, m_l = 0, m_s = +1/2)color(white)(a/a)|)))#
This set describes an electron located on the fourth energy level, in the s-subshell, in the 4s-orbital, that has spin-up.

