What is the structural difference between a 2P and a 3P orbital?
1 Answer
A
Explanation:
All
We see this in the
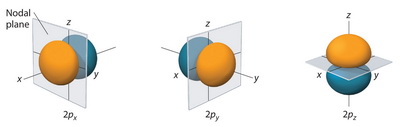
(from chemwiki.ucdavis.edu)
The
You have probably noticed that the total number of nodes in an orbital is equal to
Thus, a
Nodes can be either angular or radial.
The number of angular nodes is equal to
Since all p orbitals have
Radial nodes are spherical. The number of radial nodes is
Hence, a
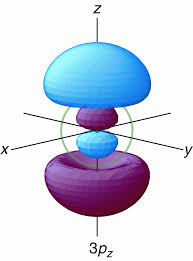
(from www.villierspark.org.uk)
Here's a computer-generated image of the three
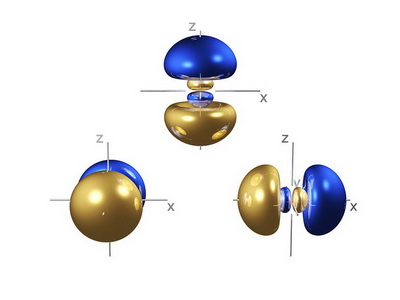
(from fineartamerica.com)
Can you see the spherical node?

