What is used to lower the energy required to make a reaction take place? It makes chemical reactions go faster without being consumed.?
1 Answer
Jul 2, 2017
Explanation:
A catalyst HAS precisely NO EFFECT on the thermodynamics of a reaction......this is chemistry not magic. They can provide an alternative reaction pathway that can alter the energy of activation for a reaction positively or negatively......
This is best appreciated by reference to graphs..
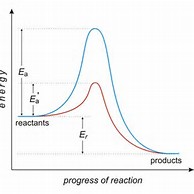
The enthalpy change of the catalyzed versus uncatalyzed reaction is PRECISELY the same. The activation energy of the catalyzed reaction is much lower, which means that a greater proportion of reactant molecules have the requisite activation energy, and the reaction proceeds faster.

