What The half-life of potassium-40 is about 1300 million years. After for half lives have passed, how much of the original sample will be left?
1 Answer
Half-life (t½) is the amount of time required for a quantity to fall to half its value as measured at the beginning of the time period.
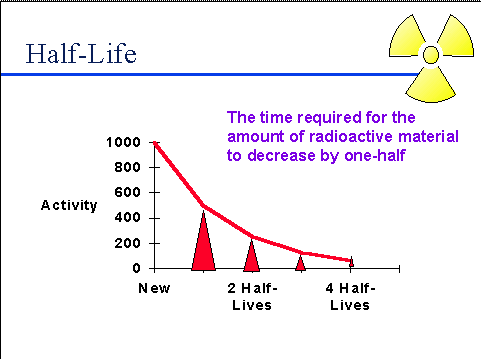
In this question (t½) of K-40 is 1300 million years , which means that after 1300 million years half of the sample would have decayed and half would be left as it is. Let us start with 200g of the sample
After 1300 million years ( first half life) 200 /2 = 100 g decays and 100 g remains left.
After another 1300 million years ( two half lives or 2600 million years) 100 /2 = 50 g decays and 50 g remains left .
After another 1300 million years ( three half lives or 3900 million years) 50 /2 = 25 g decays and 25 g remains left.
After another 1300 million years ( four half lives or 5200 million years) 25 /2 = 12.5 g decays and 12.5 g remains left.
after four half lives or 5200 million years, 12.5 g of K-40 will be left.

