What would the C-C-C bond angles be in a planar version of cyclohexane?
1 Answer
Jan 5, 2016
The
Explanation:
A planar cyclohexane would look like a regular hexagon.
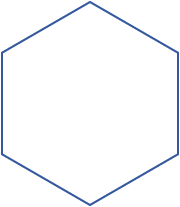
It has six sides and six interior angles
A theorem from geometry states that, for a regular polygon,
∴

Each

