When gasoline burns, it reacts with oxygen in the air and forms carbon dioxide and water vapour. How does the potential energy of the gasoline and oxygen compare with the potential energy of the carbon dioxide and water vapour?
1 Answer
How does the potential energy of a boulder on top of a cliff compare with the potential energy of the boulder on the bottom of the cliff?
Explanation:
Clearly the boulder on top of the cliff has MORE potential energy than the boulder on the bottom; and should it be pushed off the cliff edge, its potential energy will be converted into kinetic energy, to be finally dissipated as heat and sound upon its impact at the bottom of the cliff.
Now you gots
And we typically look at a graph of potential energy versus reaction coordinate.....
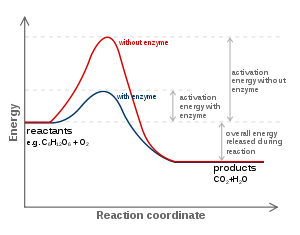
If there were incomplete combustion, which would involve the formation of

