When you have a doublet of doublets how many neighboring Hydrogens will there be?
2 Answers
There is one neighbouring hydrogen in a distinct chemical environment.
Explanation:
In the first order spectrum, for which I will add an illustration later, the given absorption is split into a doublet by coupling to the first proton, and this doublet is split into another doublet by coupling to the SECOND chemically non-equivalent proton. Sometimes these doublets overlap to give what seems to be an apparent triplet. Such so-called multiplets can be resolved by doing the experiment on a higher field instrument.
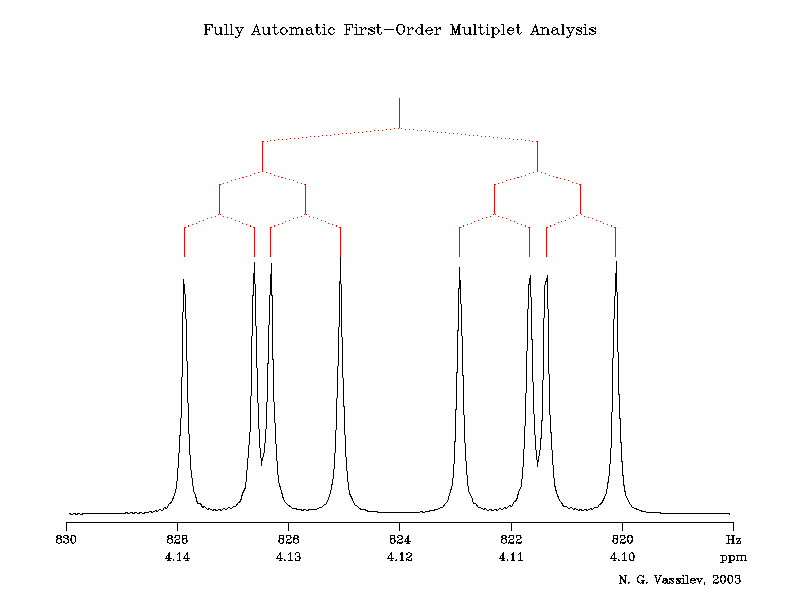
There will be two neighbouring hydrogens.
Explanation:
A doublet of doublets (dd) occurs when a hydrogen atom is coupled to two non-equivalent hydrogens.
An example is the NMR spectrum of methyl acrylate.
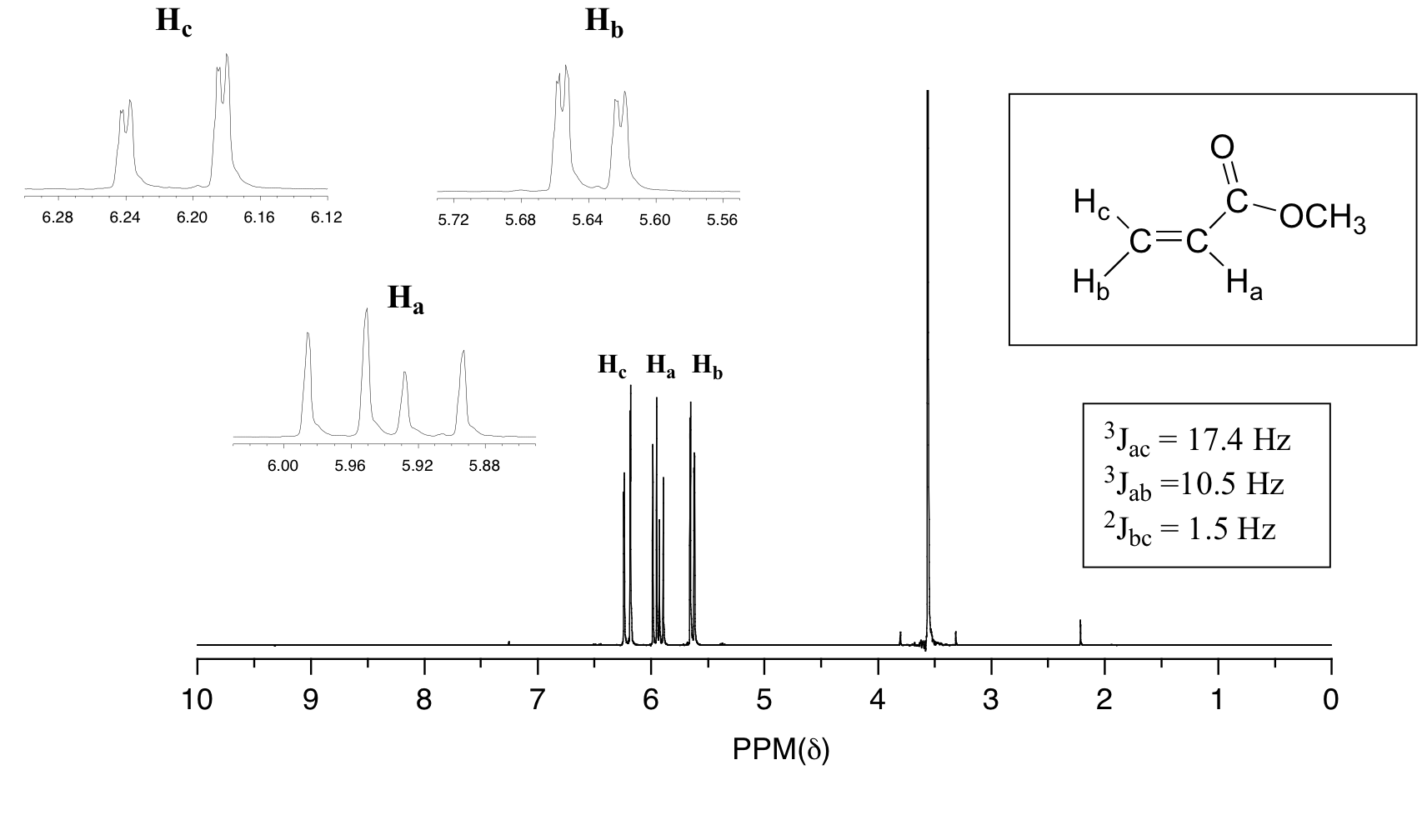
(From Chemistry LibreTexts)
Each of the vinyl protons
Let's examine the

There are four separate peaks because
The result is a doublet of doublets.

The splitting diagram shows that
These peaks are each split into doublets by coupling with



