Which of the following compounds exhibits only dispersion and dipole-dipole intermolecular interactions ? A) HBr B) CO2 C) H2O D) N2
1 Answer
The answer is A) HBr.
Since all compounds exhibit weak van der Waals interactions, the criterium to go by will be the presence of dipole-dipole interactions.
Dipole-dipole interactions take place in polar molecules, i.e. molecules that have a permanent dipole moment.
As a result, your compound must be polar and not form hydrogen bonds.
So, starting with hydrobromic acid,
Carbon dioxide is a nonpolar molecule because it has a linear molecular geometry, the two carbon-oxygen dipole moments cancelling each other out.
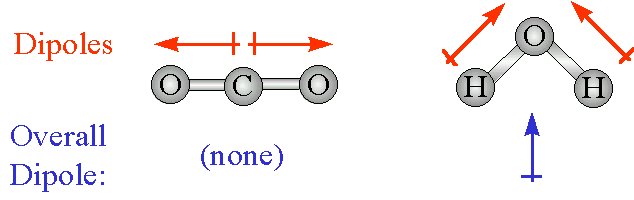
Water is a polar molecule that has a permanent dipole moment, and thus exhibits dipole-dipole interactions, but it also form hydrogen bonds, so this is not he molecule you're looking for.
Finally, the nitrogen molecule is nonpolar because the bond between the two nitrogen atoms is a perfect covalent bond.