Which one of the following is the ground-state electron configuration of the Mg2+ ion?
1 Answer
Apr 4, 2015
Magnesium is located in period 3, group 2 of the periodic table, and has an atomic number equal to 12. This means that a neutral magnesium atom has 12 electrons that surround its nucleus.
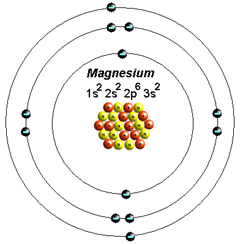
When magnesium loses two of its electrons, it becomes the
As a result, the electron configuration of the
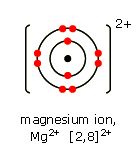
The

