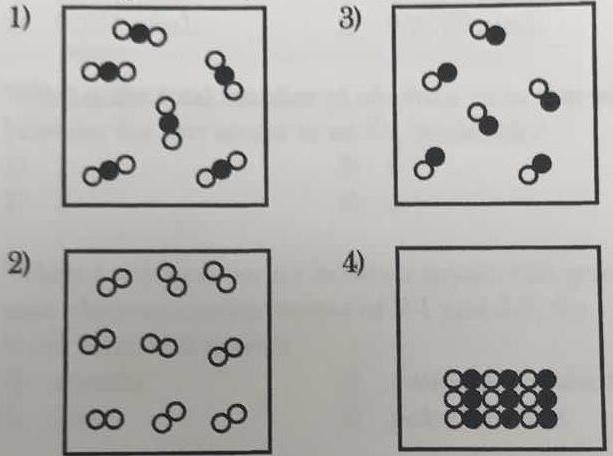
Which particle diagram represents a sample containing the compound #CO_2#? How do you determine this?
1 Answer
Dec 30, 2016
Well, for a start the diagram represents a gas, so (4) is a non-starter..........
Explanation:
(4) is eliminated immediately. Why? Which of (1), (2), and (3) best represents
I think (I) is the clear choice. (2) might represent a homonuclear, diatomic molecule such as
Given the representations in the diagrams, how would you represent the (bent) molecules

