Which sublevel is filled after the 5s sub level?
1 Answer
Feb 14, 2016
The 4d sublevel is filled next, after the 5s sublevel.
Explanation:
Electrons fill the sub levels in energy order:
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
If we add the number of electrons that each sublevel holds it looks like this:
Source: http://www.kentchemistry.com/links/AtomicStructure/Sublevels.htm
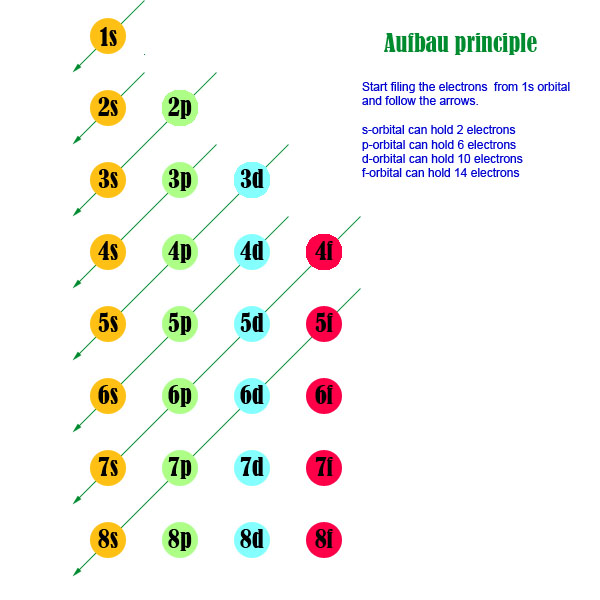
The Aufbau diagram can be memorized more easily than the electron configuration of all the sublevels up to


