Why are atoms and molecules always in motion?
1 Answer
They are not always in motion. They are always in motion if and only if the temperature of the surroundings is greater than absolute zero (
When that is the case, the average kinetic energy is nonzero, and thus, the molecules have the energy to "vibrate" (stretch and bend bonds) and rotate, while atoms have the energy to move linearly in three dimensions (temperature is derived from the average kinetic energy, thus nonzero temperature implies nonzero average kinetic energy).
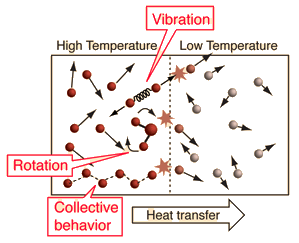
(As a fun fact, the molecular vibration can be detected by infrared spectroscopy if the molecule is asymmetrically vibrating.)
Because energy is conserved in the universe, any energy the molecule loses is regained in some other way, such as the transfer of energy from other molecules that had more energy (having an average kinetic energy implies that the individual energies can be different).
So, if at least one molecule has energy, it can redistribute energy into other molecules and keep them moving as well.

