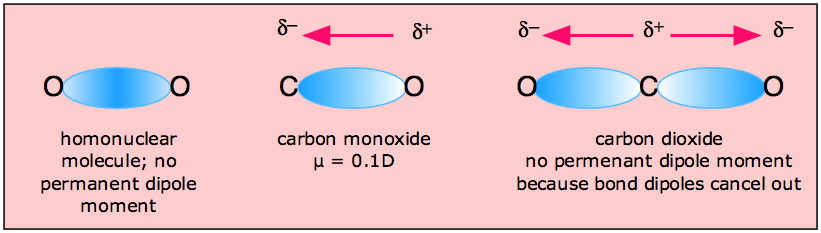
Why are electrons shared equally in oxygen, #O_2# but not in carbon monoxide #CO#?
2 Answers
Explanation:
We can answer this question by looking at the polarity of both molecules.
Oxygen,
In a non polar molecule, the electron density is equally distributed over the atoms making the molecule.
However,
In a polar molecule, the electron density is pulled by the more electronegative atom (which is oxygen), and therefore, the electron density is not distributed evenly on both atoms of the molecule
Refer to the Explanation.
Explanation:
Every element has a property called electronegativity (EN), which is the tendency of a bonded atom to attract electrons to itself. Bond character, such nonpolar and polar covalent, and ionic, is determined by the difference in electronegativities
A
http://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text7/Tx71/tx71.html
The electronegativity of an oxygen atom is 3.44. Since two oxygen atoms make up a molecule of oxygen
The electronegativity of a carbon atom is 2.55. The
https://en.wikipedia.org/wiki/Electronegativity#Pauling_electronegativity