Why are molecules formed?
1 Answer
Molecules form so that the atoms of each element in the molecule become stable..
Explanation:
Atoms form chemical bonds with one another in order to become stable, which means achieving an octet (8) of valence electrons, or in the case of hydrogen, a duet (2) of valence electrons. This means their valence shell becomes full. By achieving an octet or duet, they achieve a noble gas configuration, which makes the atoms stable.
Molecules are composed of nonmetal atoms which share valence electrons in covalent bonds so that all of the bonded atoms have an octet (or duet). This makes the atoms more stable than existing alone, which is why in nature nonmetals are not generally found as individual elements.
It is important to note that atoms bond in order to become stable. For example, they don't know that we need oxygen gas or water to live. The atoms of these molecules share valence electrons in order to achieve an octet or duet, which makes them stable.
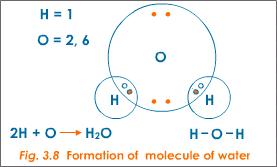
Hydrogen atoms have one valence electron, which they share to achieve a duet of valence elelctrons. The bond is the area of overlap.
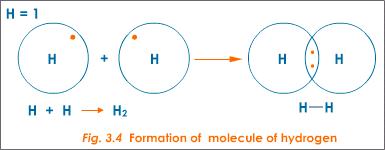
Oxygen atoms have six valence electrons. They each contribute two valence electrons to achieve an octet. In this case, the two pairs of shared electrons form a double bond. The bond is in the area of overlap.
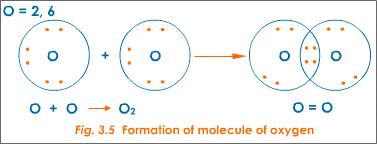
In a molecule of water, two hydrogen atoms share their single valence electrons with two valence electrons from the oxygen atom. This gives the hydrogen atoms a duet and the oxygen atom an octet. The bonds are in the areas of overlap.