Why can a meso compound have an enantiomer?
1 Answer
A meso compound CANNOT have an enantiomer.
Let me show you an example.
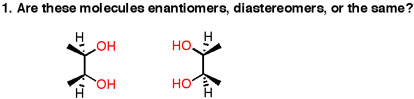 https://masterorganicchemistry.files.wordpress.com/
https://masterorganicchemistry.files.wordpress.com/
Notice how if you rotate the right-compound
How about these?
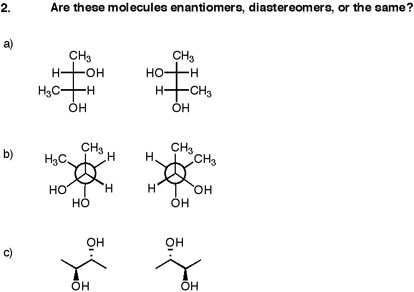 https://masterorganicchemistry.files.wordpress.com/
https://masterorganicchemistry.files.wordpress.com/
Were you fooled? If so, you might want to take a look at some of the following tricks to recognize meso...
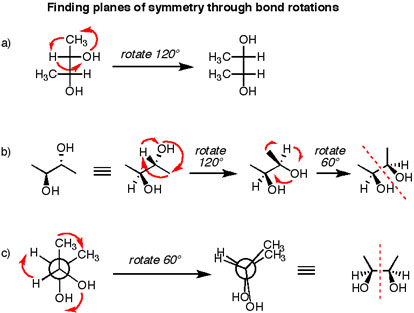 https://masterorganicchemistry.files.wordpress.com/
https://masterorganicchemistry.files.wordpress.com/
These three compounds are in fact the same compound, and they're all meso. Just drawn in different ways.
a) was a Fischer projection. But rotate the top carbon
b) was typical line notation with solid and hashed wedges. Rotate the rear by
c) was a staggered Newman projection. Turn it into an eclipsed conformation and you've got a mirror plane. Definitely meso.
Oh, by the way, all the compounds above were identical. :)
Rotate the first two compounds we looked at from the top of the page on their vertical axes toward you by

