A single bond consists of a #sigma# bond, which is defined as the head-on overlap between two compatible orbitals.
Because hybrid orbitals are made to match the symmetry of the incoming (i.e. not-yet-bonded) atom's atomic orbital for the sake of being able to bond at all (which is usually if not always "totally symmetric about the internuclear axis"), they always have to overlap head-on.
Thus, a hybrid orbital has to make a #sigma# bond.
Not just #sp^3#, but any hybrid orbital. Even in a triple bond, like in acetylene (#"H"-"C"-="C"-"H"#), the #pi# bonds are made by the #p_x# and #p_y# orbitals (or any qualified equivalent sidelong orbital overlap), while the #sigma# bonds are made with the hybrid orbitals, which consist of only the #p_z# and #s# orbitals.
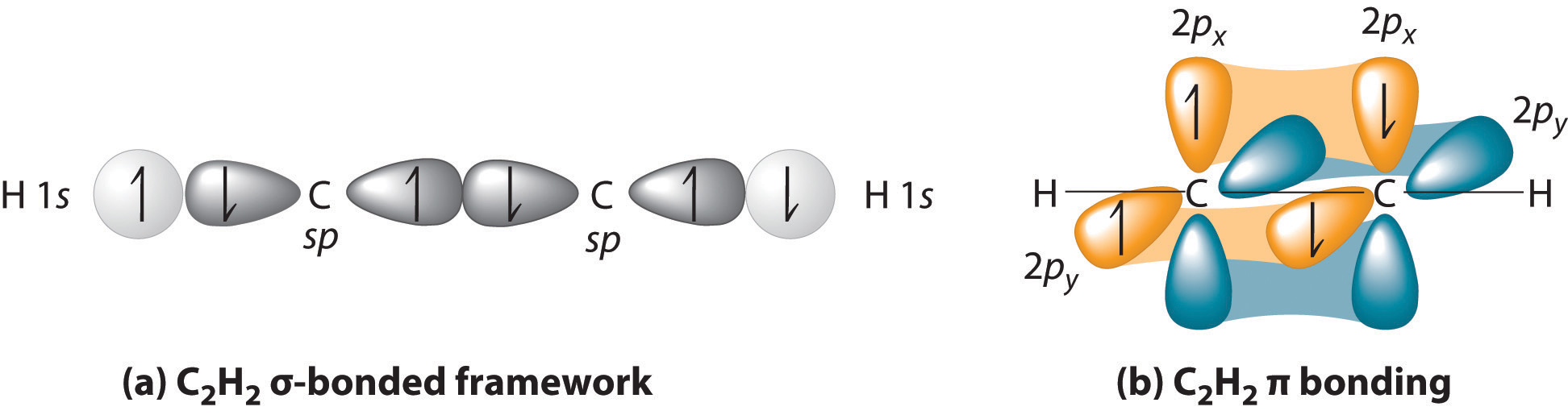
Here, the #z# axis is defined as the internuclear axis, whereas the #x# and #y# axes are vertical and towards us, respectively.
As an alternative, consider ethene (#"H"_2"C"="CH"_2#), where the double bonds lie on the #xy#-plane in the plane of the screen/paper, and the #z# axis protrudes outward towards us.
If we consider the #sp^2# hybridization of carbon in ethene, carbon actually uses three #sp^2# hybrid orbitals: one each to #sigma# bond with the hydrogens, and one to #sigma# bond with the other carbon. (You would get that if you drew the molecule with all single bonds and no double bonds.)
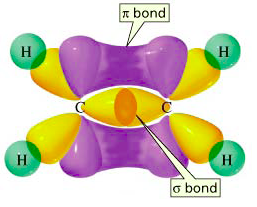
It made each hybrid orbital by mixing the #2s#, #2p_x#, and #2p_y# orbitals together and distributing their angular positions evenly on the #xy#-plane since the original #2p# orbitals used to construct them were actually aligned in the #x# and #y# directions, respectively. (The notation #sp^2# comes from mixing one #s# and two #p# orbitals together to get #33%# #s# character and #66%# #p# character.)
Hence, the #\mathbf(sigma)# bonds of ethene (shown in yellow) lie on a single plane.
In addition, we have the #\mathbf(2p_z)# atomic orbital (perpendicular to the plane of the molecule, in purple) from each carbon that is used to #\mathbf(pi)# bond (sidelong overlap) with the other carbon, thus constructing the second component of the double bond (one #sigma# + one #pi# bond = one double bond).
It was not used in the orbital hybridization, and it remains as a different, incompatible orbital (with respect to the #\mathbf(2p_x)# and #\mathbf(2p_y)#) for #\mathbf(sigma)# bonding within the molecule. The only thing it can do at this point is #pi# bond because it is oriented precisely to do so.



