Why does a catalyst help a reaction proceed at a faster rate?
1 Answer
Oct 27, 2016
Because it provides an alternative reaction pathway.
Explanation:
Because (usually) the alternative reaction pathway has a lower activation energy, more reactant molecules have the requisite energy to overcome the energy barrier, and a faster rate of reaction results. Note that a catalyst has NO effect on the thermodynamics of a reaction.
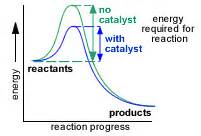
The diagram (which I hope I have attributed properly!) clearly shows that the thermodyamics of the reaction are the same whether it is catalyzed or uncatalyzed. The kinetics of the reactions are manifestly different.

