Why does ammonia have 4 shells that are sp3 hybridized, rather than having the s-shell for the non-bonding electron pair and the remaining 3 p-shells used for the covalent bonding with the hydrogen atoms?
1 Answer
Since all three hydrogens are identical, but four electron groups are present in
AMMONIA LOOKS OFF WITH PURE ORBITALS
Suppose we did have one
That would actually imply that
but we know that's not right.
RECONCILING THE NOTION OF WHAT AMMONIA LOOKS LIKE
Here are the premises we're working with:
- Nitrogen has its
#2s# ,#2p_x# ,#2p_y# , and#2p_z# orbitals to use. - Its
#2p_x# orbital is aligned precisely along the#x# axis. - Its
#2p_y# orbital is aligned precisely along the#y# axis. - Its
#2p_z# orbital is aligned precisely along the#z# axis. - Since ammonia is three-dimensional, it requires
#sp^3# hybridization to make its bonds. We'll prove that in a moment.
To make a bond with three identical hydrogens AND have a fourth electron group for a lone pair, we need three dimensions, AND we need to move the orbitals off the cartesian axes!
However, if any of the
A pure orbital cannot be re-aligned onto a different axis without simply becoming a different orbital.
The quantum mechanical reason why these directional pure orbitals must stay on the axes for which they were defined is that they must maintain orthogonality (perpendicularity) to be physically representable with quantum mechanical equations.
EFFECT OF HYBRIDIZATION ON ORBITAL DIRECTION
NOTE: This is just a proof, so you do not need to know how to do this; just follow it and work to understand the conclusion.
One of the major effects of hybridization is that it allows directional orbitals like the
If we represent the
#vec(2s) = << 0,0,0 >># #vec(2p_x) = << 1,0,0 >># #vec(2p_y) = << 0,1,0 >># #vec(2p_z) = << 0,0,1 >>#
If you perform the dot product with any two of these, you would get
If you hybridize only three of these pure orbitals to get
#vec(2s) + vec(2p_x) + vec(2p_y) = << stackrel(x)overbrace(1),stackrel(y)overbrace(1),stackrel(z)overbrace(0) >>#
which is a two-dimensional vector on the
Almost there, but not quite, because we're missing the trigonal pyramidal structure (the
#vec(2s) + vec(2p_x) + vec(2p_y) + vec(2p_z) = << stackrel(x)overbrace(1),stackrel(y)overbrace(1),stackrel(z)overbrace(1) >>#
which is a three-dimensional vector, and thus can be freely rotated in any direction in three dimensions and still be a valid orbital under quantum mechanical restrictions.
(These orbitals can be scaled in any dimension as well, so we're not restricted to an orbital confined within a
A NOTE ABOUT SYMMETRY
The other reason why hybridization is necessary for ammonia is that all three identical hydrogen atoms that come in to bond need to bond identically.
A
To make the
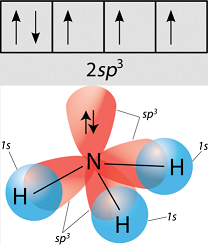
(A general chemistry textbook probably wouldn't explain why, so you don't really need to know the nitty gritty details for this caveat.)

