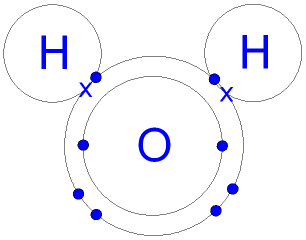
Why Hydrogen can only share 1 pair of electrons?
1 Answer
Dec 18, 2016
See the explanation.
Explanation:
The hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond.
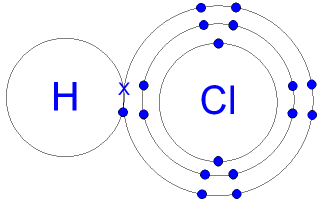
The following diagram shows the structural formula for hydrogen chloride. You can see that the hydrogen atom can form only one covalent bond with the chlorine atom.
The following diagram shows the structural formula for water,