Why is Boron Trifluoride written in two ways in the lewis dot diagram structure?
The picture immediately below these sentence makes the most sense to me. Boron gets an octet of 8 and the respective Fluorides get their octet of 8. The second picture is weird and Boron is not complete, why is this correct It’s on google images too!
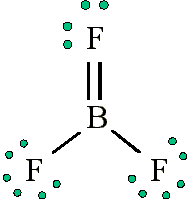
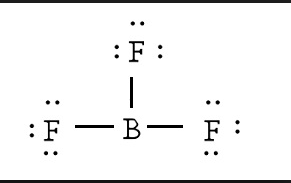
The picture immediately below these sentence makes the most sense to me. Boron gets an octet of 8 and the respective Fluorides get their octet of 8. The second picture is weird and Boron is not complete, why is this correct It’s on google images too!

2 Answers
Because experiments suggest that there is some degree of overlap....
Explanation:
So
Of course,
Many boron chemists use such reagents as
The 2nd structure is correct because:
Explanation:
To make a Lewis structure we calculate the formal charge( Its a charge on every element in Lewis structure which helps to determine us whether the structure is correct or not according to the rules.the total formal charge should come as the total charge on the molecule. Its formula is = [no. of valence electrons on atom] – [non-bonded electrons + number of bonds]. or a simple one{if no lone pair are there} = 1/2 no. of bonds.)on each atom.
Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything.
(in fig. 1). We must examine the formal charges of this structure. The fluorine that shares a double bond with boron has six electrons around it . This is one less electron than the number of valence electrons it would have naturally, so it has a formal charge of +1. The two flourines that share single bonds with boron have seven electrons around them (six from their three lone pairs and one from their single bonds with boron). This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Finally, boron has four electrons around it (one from each of its four bonds shared with fluorine). This is one more electron than the number of valence electrons that boron would have on its own, and as such boron has a formal charge of -1.
The first structure contradicts one of the major rules of formal charges: Negative formal charges are supposed to be found on the more electronegative atom(s) in a bond, but in the structure depicted , a positive formal charge is found on fluorine, which not only is the most electronegative element in the structure, but the most electronegative element in the entire periodic table.
Boron on the other hand, with the much lower electronegativity of 2.0, has the negative formal charge in this structure. This formal charge-electronegativity disagreement makes this double-bonded structure impossible.


