Why is "BrF"_4^(-) square planar, whereas "BF"_4^(-) is tetrahedral?
1 Answer
Br has 7 valence electrons in its ground state electron configuration where as B only has three.
Explanation:
If you look at the positions of Br and B on the periodic table, they have ground state electron configurations of the following:
For Br,
For B,
Looking at these electron configurations, it can be seen that the valence shell of bromine (n=4) has 7 electrons, 2 from the s subshell, and 5 in the p subshell.
Since Br is the central atom, and is bonded to four F atoms (who also have 7 valence electrons), 4 of the 7 electrons in the valence shell of Br will be bonded to an F atom. This means that 3 electrons will not be in a bond. Since the compound is negatively charged, this means the additional electron (carrying the negative charge) will be paired with on of the 3 lone electrons.
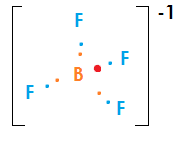
Drawing this structure out, you will see that the four F's will align in a square plane, and lone pairs will be on either side of the plane. Hence, square planar, as shown below where green dots came from Br, blue from F, and red is the extra electron providing the overall negative charge.
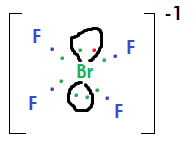
Looking at
Again, an extra electron shown in red that is not present in the ground state configurations will provide the negative charge (shown in red)