Why is cyclopropane much more reactive than cyclohexane?
1 Answer
Nov 20, 2015
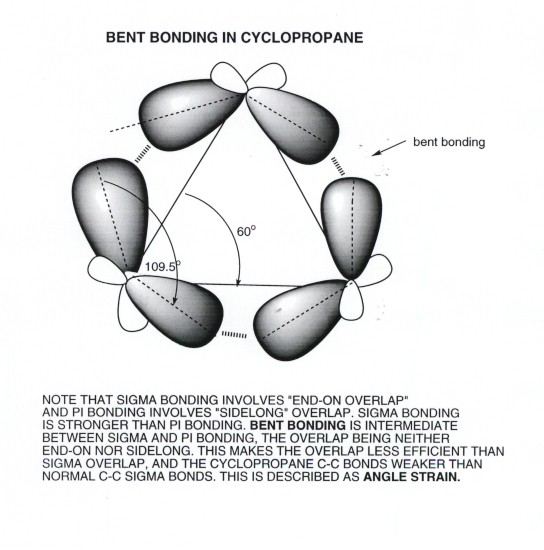
Because of ring strain. Can you imagine that cyclopropane would have a lot of spatial crowding from the orbitals? Fortunately, the orbitals bend a little to compensate, but that isn't enough to completely stabilize it.
This makes it reactive because it really wants to break those bonds and unbend the orbitals. Similarly, if you were lying down, wouldn't you want to spread out your arms and legs, rather than curling up into a ball as you sleep? It's more comfortable.
 http://research.cm.utexas.edu/
http://research.cm.utexas.edu/
Cyclohexane doesn't have this problem. It can assume a chair conformation just fine.