Why is hydrogen bonding important?
1 Answer
Hydrogen bonding is important because it is crucial to all life on Earth.
Here are three reasons why hydrogen bonding is important.
1. The structure of DNA
DNA has a double-helix structure because hydrogen bonds hold together the base pairs in the middle. Without hydrogen bonds, DNA would have to exist as a different structure.
2. The specific heat capacity/ boiling point of water
Water has a relatively high boiling point due to hydrogen bonds. Without hydrogen bonds, water would boil at about -80 °C. Water in oceans and lakes would rapidly boil away. This would cause massive problems for life on Earth.
3. The structure of proteins
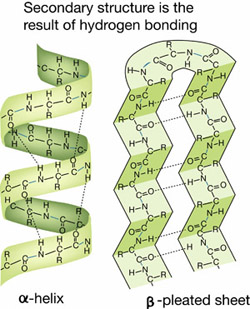
Hydrogen bonds are important in forming the secondary structures of proteins —the helix and the pleated sheet.
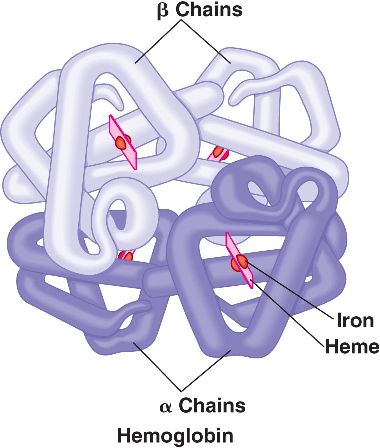
The hemoglobin molecule consists of four subunits. The proteins in the subunits are coiled into helices that are held together by hydrogen bonds. Without the hydrogen bonds to keep its shape, hemoglobin would be unable to function.