Why is infrared spectroscopy a non-destructive technique?
1 Answer
Today, FTIR ( Fourier Transform Infrared) Spectroscopy is the common method for IR Spectroscopy. The instrumentation looks roughly like this:
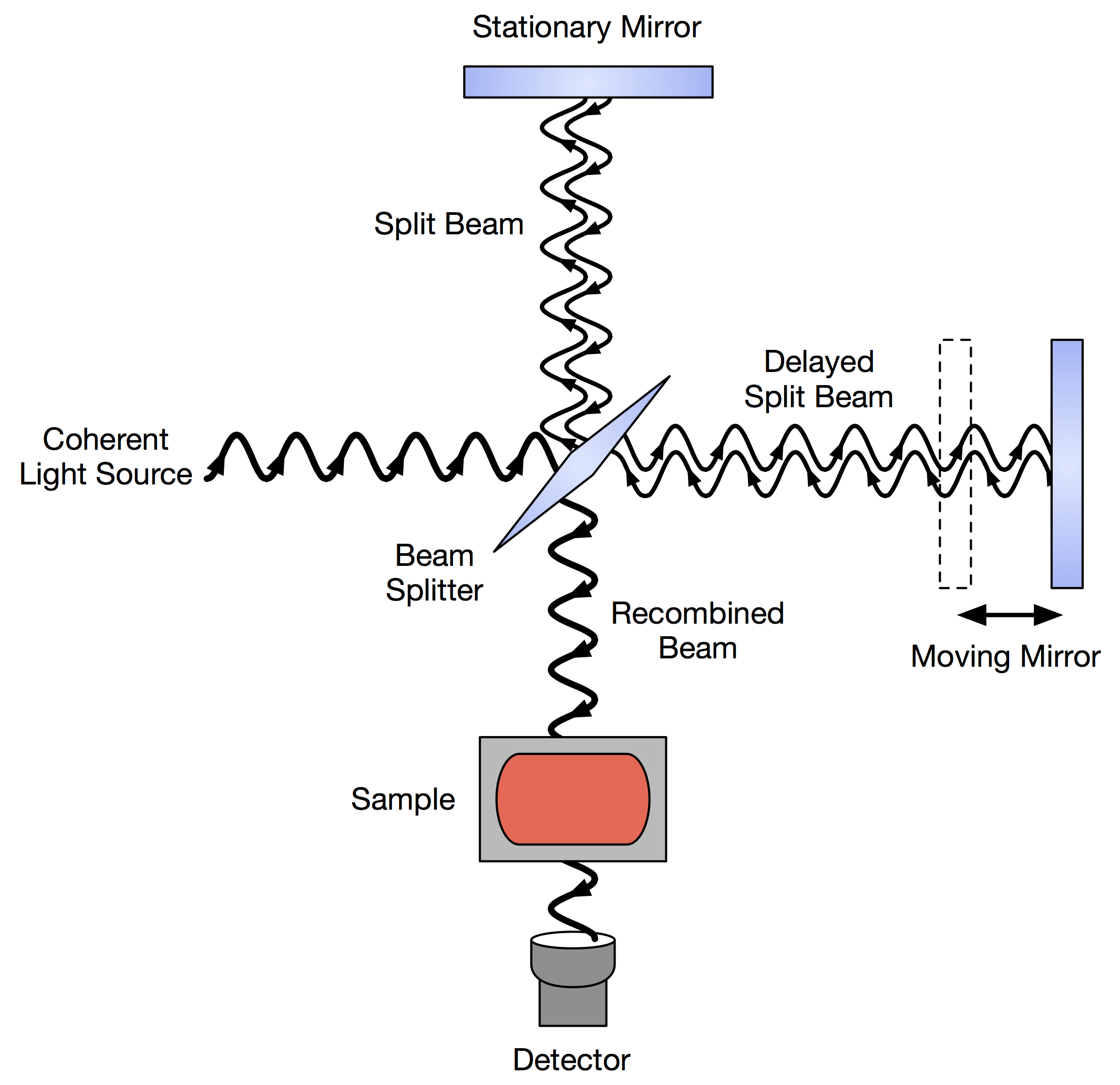
A coherent light source can be something like a laser, which is a concentrated, uniform light source (coherent means uniform). And you know how a mirror works!
From this diagram, all you really need to understand is that light essentially passes through surfaces (mirrors or beam splitters) that either reflect or split the light, with controlled phase cancellation due to the constant-velocity moving mirror. Then it passes through the sample.
But the light doesn't fragment the sample; it just excites it at various frequencies so that the molecule vibrates at certain intensities depending on how close the current frequency is to the resonant frequency.
The closer to the resonant frequency, the stronger the peak. Based on the direct relationship between vibration intensity and IR peak intensity, the peak intensity varies accordingly.
Bottom line, the light does not fragment the sample, and so it is non-destructive.
(One spectroscopy technique that involves destructive sampling may include "primitive" Mass Spectroscopy fragmentation techniques which involve hard ionization, for example, but these days Mass Spec has improved to the point where it's not as much of an issue.)

