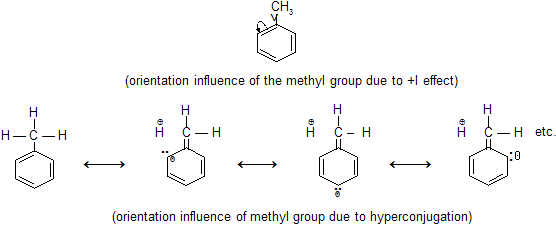
Why is methyl benzene more reactive than benzene?
1 Answer
Mar 9, 2016
Due to +I effect of Methyl Group
Explanation:
Methyl group has got electron repelling property due to its high
+ I effect caused by hyper conjugation . That is why it pushes electron towards benzene ring thus the benzene ring in toluene molecule becomes activated for having higher density of negative charge compared to simple benzene molecule. This makes the toluene molecule susceptible to electrophilic attack . That is why toluene shows more re-activity than benzene