Why is the IR spectrum inverted?
1 Answer
It's arbitrary.
You can feel free to invert it again and switch between absorbance ("right-side-up") and %transmittance ("upside-down") before you print one out.
It really doesn't matter whether the peaks are facing downwards or upwards, or whether the wavenumbers are left-to-right or right-to-left; it communicates the same information in slightly different ways.
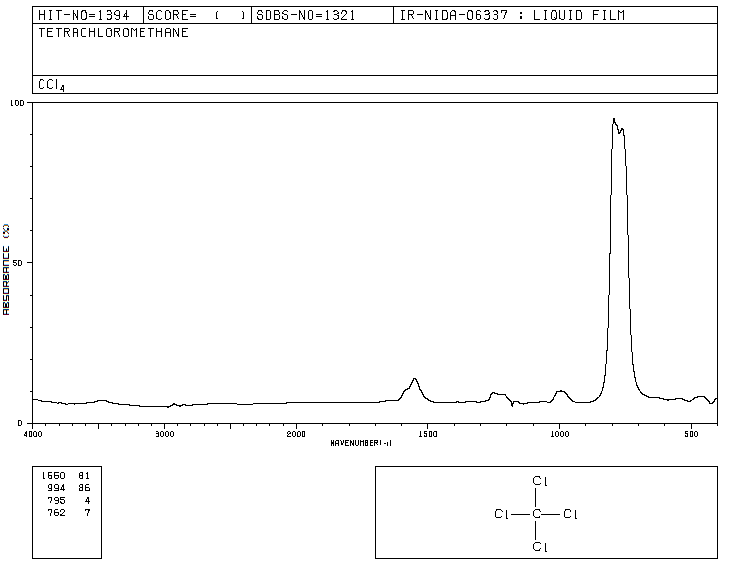
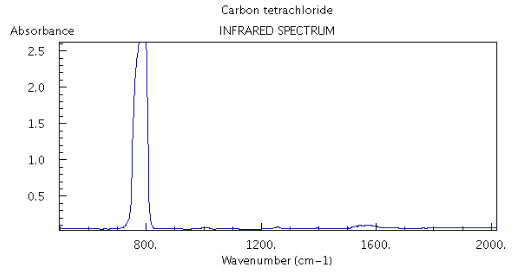
Honestly, I've actually submitted lab reports where I've used absorbance or %transmittance, and there were no complaints either way (just as long as I didn't do both in the same report).
As for the wavenumbers going right to left, it's just a common convention. There's no real reason to do that, given that it's been done both ways.
What matters is what the peaks look like. In other words:
- how strong/weak they are
- how narrow/wide they are
- where they are horizontally (their vibrational frequency)
because that's how you identify which bond stretches or bends those rotational or vibrational peaks correspond to.
You just need to know what the peak looks like, not which way it's facing.

