Why is there a mass increase from the reactants to the products in the following nuclear reaction?
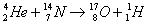
1 Answer
Since in nuclear reactions, the mass-energy is conserved, therefore, there no mass increase from reactants to products in this reaction as well.
Explanation:
This is a classical example of first man-made nuclear reaction carried out successfully by Rutherford in 1919. He bombarded various with with alpha particles and observe that once in a while alpha particle would hit the nucleus of an atom and disarrange it.
He experimented and found that alpha particle could knock protons out of nitrogen nuclei and thereafter merge with what remained.
The reaction looked like in terms of mass number of reactants and products as
Nitrogen-14
We need to remember that three conservation laws that apply to nuclear reactions are
- The number of nucleons is conserved.
- The charge is conserved, or the sum of the charges of reactants is equal to the sum of the charges of products.
- The mass-energy is conserved.
Consequently the above nuclear reaction can be written as
We see from this equation that conditions 1. and 2. are met. For the 3. condition.
Binding energy /Mass defect consideration.
Some reading material is here.
If we go through the average binding energy per nucleon curve as below
and observe that mass-energy in the above nuclear reaction is also conserved.
A very good match given that
